Scientific Achievement
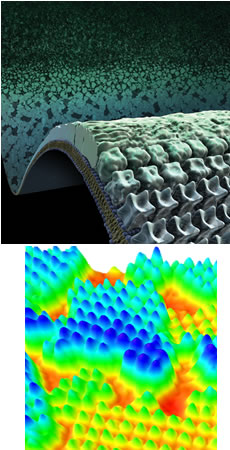
Bottom: 3-dimensional interpretation of AFM data acquired during analysis.
Using in-situ Atomic Force Microscopy to directly observe S-layer formation on mica chips, Foundry scientists show that a kinetic trap occurs during protein self-assembly. Some domains become trapped in high-energy states. Once in this state, the energy barrier for relaxation into low-energy domains approaches that required for initial formation.
Significance and Impact
This study of S-layer crystallization demonstrates the role of kinetic traps in determining the pathway of ordered systems. Monomers rapidly cluster together in various intermediate conformations, before relaxing into stable states. Because protein architectures require conformational changes from individual monomers, this phenomenon may be generally applicable to protein self-assembly.
Research Details
- Using in situ AFM, demonstrated that the S-layer forms two types of compact crystalline domains on mica, a stable, low-energy tall phase, and an intermediate, high-energy, short phase.
- Time and temperature analyses revealed minimal difference between energy required for formation of the two states, but if the higher-energy state forms, the barrier to transformation to the low-energy state is 25 kT.

