Adapted from this Berkeley Lab Press Release
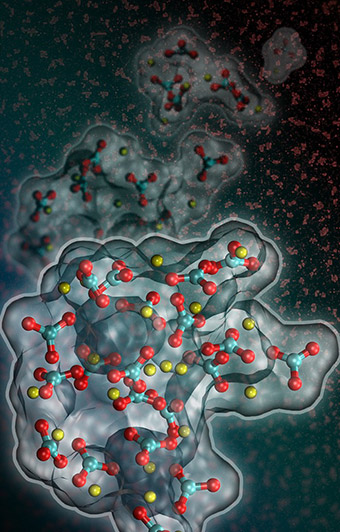
Computer simulations conducted at the Molecular Foundry could help scientists make sense of a recently observed and puzzling wrinkle in one of nature’s most important chemical processes. It turns out that calcium carbonate—the ubiquitous compound that is a major component of seashells, limestone, concrete, antacids and myriad other naturally and industrially produced substances—may momentarily exist in liquid form as it crystallizes from solution.
Calcium carbonate is a huge player in the planet’s carbon cycle, so any new insight into how it behaves is potentially big news. The prediction of a dense liquid phase during the conversion of calcium carbonate to a solid could help scientists understand the response of marine organisms to changes in seawater chemistry due to rising atmospheric CO2 levels. It could also help them predict the extent to which geological formations can act as carbon storage reservoirs, among other examples.
The research is published in the August 23 issue of the journal Science. It was performed in support of the Center for Nanoscale Control of Geologic CO2, an Energy Frontier Research Center established at Berkeley Lab by the U.S. Department of Energy.

