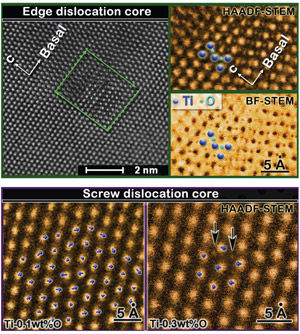
Scientists working at the Molecular Foundry have found the mechanism by which titanium, prized for its high strength-to-weight ratio and natural resistance to corrosion, becomes brittle with just a few extra atoms of oxygen.
The discovery, led by the Foundry’s Andrew Minor, who also serves as a professor at UC Berkeley, has the potential to open the door to more practical, cost-effective uses of titanium in a broader range of applications. The popular silver-gray metal can already be found in high-end bicycles, laptops and human implants, among other products. But high-grade titanium with low levels of oxygen is hard to come by, and the expense of purifying the metal has prevented its wider use in applications for the construction, automotive and aerospace industries.
Minor led a research team that focused on solving the long-standing mystery in metallurgy of how oxygen causes such a profound change in the characteristics of metals. “Oxygen is like poison to titanium,” said Minor. “With more oxygen, the material gets harder and more susceptible to cracks, qualities that are not desirable for structural materials.”

