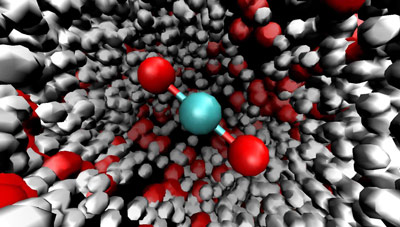
Blink your eyes and it’s long gone. Carbonic acid exists for only a tiny fraction of a second when carbon dioxide gas dissolves in water before changing into a mix of protons and bicarbonate anions. Despite its short life, however, carbonic acid imparts a lasting impact on Earth’s atmosphere and geology, as well as on the human body. However, because of its short lifespan, the detailed chemistry of carbonic acid has long been veiled in mystery. Researchers the Molecular Foudnry are helping to lift this veil through a series of unique experiments. In their latest study, they’ve shown how gaseous carbon dioxide molecules are solvated by water to initiate the proton transfer chemistry that produces carbonic acid and bicarbonate.
This work follows a separate recent study in which the hydration structure of carbonic acid itself was characterized. Ultimately, such studies will lead to a complete understanding of how atmospheric carbon dioxide is captured and transformed by ocean surfaces, a crucial role in the carbon cycle. They will also enable us to address how bicarbonate anions interact with calcium and magnesium cations in solution to create the nanoclusters that nucleate limestone formation, and how bicarbonate anions buffer blood and other bodily fluids.

