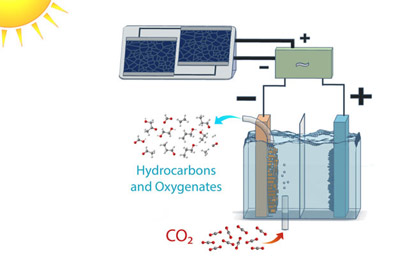
Molecular Foundry users have harnessed the power of photosynthesis to convert carbon dioxide into fuels and alcohols at efficiencies far greater than plants. The achievement marks a significant milestone in the effort to move toward sustainable sources of fuel.
Many systems have successfully reduced carbon dioxide to chemical and fuel precursors, such as carbon monoxide or a mix of carbon monoxide and hydrogen known as syngas. This new work, described in a study published in the journal Energy and Environmental Science, is the first to successfully demonstrate the approach of going from carbon dioxide directly to target products, namely ethanol and ethylene, at energy conversion efficiencies rivaling natural counterparts.
The researchers did this by optimizing each component of a photovoltaic-electrochemical system to reduce voltage loss, and creating new materials when existing ones did not suffice.
Researchers engineered a complete system to work at different times of day, not just at a light energy level of 1-sun illumination, which is equivalent to the peak of brightness at high noon on a sunny day. They varied the brightness of the light source to show that the system remained efficient even in low light conditions.
When the researchers coupled the electrodes to silicon photovoltaic cells, they achieved solar conversion efficiencies of 3 to 4 percent for 0.35 to 1-sun illumination. Changing the configuration to a high-performance, tandem solar cell connected in tandem yielded a conversion efficiency to hydrocarbons and oxygenates exceeding 5 percent at 1-sun illumination.
The researchers characterized the materials at the Molecular Foundry. The results helped them understand how the metals functioned in the bimetallic cathode. Specifically, they learned that silver aids in the reduction of carbon dioxide to carbon monoxide, while the copper picks up from there to reduce carbon monoxide further to hydrocarbons and alcohols.

