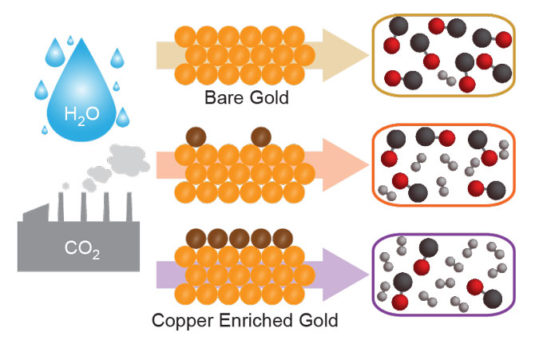
Molecular Foundry users have developed a new recipe for creating synthesis gas mixtures, or syngas, that involves adding a pinch of copper atoms sprinkled atop a gold surface. The new material supports a room-temperature electrochemical reaction that can convert carbon dioxide and water into syngas, a mixture of carbon monoxide and hydrogen, and an important precursor in the production of chemicals and synthetic fuels.
The researchers say syngas can be converted downstream into small molecules, like ethanol, or larger hydrocarbons, such as those in gasoline, by fermentation or thermochemistry. Designing a material and a process that can easily control the composition of syngas would be an important improvement in reducing the environmental impacts of those industrial processes.
They describe their design in a paper recently published in the Journal of the American Chemical Society. The study was led by Peidong Yang, senior faculty scientist at the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) Materials Sciences Division, and Edward Sargent, professor at the University of Toronto’s Department of Electrical and Computer Engineering.
The researchers found that they could control the amount of carbon monoxide and hydrogen generated by the electrocatalyst by adjusting the amount of copper atoms layered onto a nanostructured gold surface.

