Foundry industry users developed a multinozzle emitter array (MEA), a silicon chip that can dramatically shorten the time it takes to identify proteins, peptides, and other molecular components within small volumes of biological samples. This patented technology is now being commercialized by Newomics Inc., to further develop the product and build a platform for personalized health care. Some of the early work on multinozzle emitters was done at the Molecular Foundry.
Newomics’ product, which is based on the core technology developed at Berkeley Lab, is designed to work with mass spectrometers, a machine commonly used by research scientists, the pharmaceutical industry, and increasingly in clinical labs, to measure the structure and concentration of molecules. Once molecular parts are isolated, scientists can begin to understand how they work together as a system, a field known as systems biology, which holds great promise for better medicines and diagnostics as well as a host of other applications.
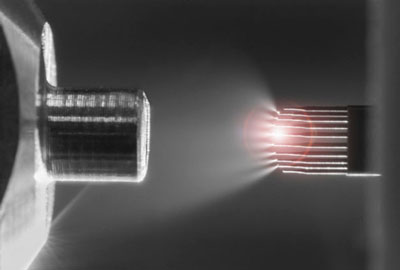
The dominant method for analyzing biological molecules such as peptides and proteins in a complex mixture is electrospray ionization mass spectrometry (ESI-MS), a technique in which molecular samples are delivered to the machine as an ionized mist, propelled by an electric current. But there is a bottleneck at the front end of ESI-MS, making it slow and expensive. Each sample has to be loaded, lined up, and sprayed one at a time.
Instead of a single capillary, Newomics’ M3 emitter has eight or more nozzles working together to split a single large flow into smaller flows. For the MEA, up to 96 M3 emitters are packaged on a single chip. The development of these technologies involved a blend of microfluidic, microelectronic, and electrochemical innovations.
By clearing up the bottleneck and increasing throughput, Newomics’ emitter could dramatically reduce the cost of testing each sample. And by improving the sensitivity, it will also be possible to detect very low concentrations of molecules. For example, they showed they could analyze many different modified forms of proteins such as glycated albumin and apolipoproteins, in addition to the conventional glucose and HbA1c in diabetes monitoring, using a single drop of blood. Such tests have the potential to enable better long-term monitoring and disease management of diabetes.

