Scientists have discovered a novel chemical state of the element manganese. This chemical state, first proposed about 90 years ago, enables a high-performance, low-cost sodium-ion battery that could quickly and efficiently store and distribute energy produced by solar panels and wind turbines across the electrical grid.
This direct proof of a previously unconfirmed charge state in a manganese-containing battery component could inspire new avenues of exploration for battery innovations. The study was led by researchers at Natron Energy, formerly Alveo Energy, a Palo Alto, California-based battery technology company.
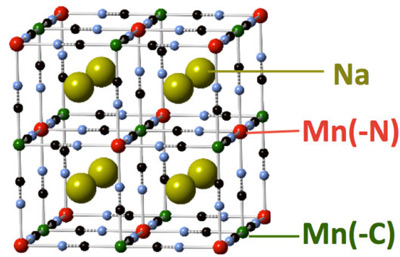
The battery that Natron Energy supplied for the study features an unconventional design for an anode, which is one of its two electrodes. Compared with the relatively mature designs of anodes used in lithium-ion batteries, anodes for sodium-ion batteries remain an active focus of R&D.
Both of the battery’s electrodes utilize the same type of materials based on elements known as “transition metals” that are useful in chemistry because they can exhibit various charged states. The other electrode, called a cathode, contains copper, nitrogen, carbon, and iron.
The battery has been shown to deliver up to 90 percent of its total energy in a very fast, five-minute discharge, and to retain about 95 percent of its discharge capacity for 1,000 cycles. It offers an alternative to gravity-based energy storage systems for electric grid, in which water is pumped uphill and then released downhill on demand to generate electricity.
The Natron Energy researchers studied the battery materials at the Molecular Foundry, and then studied some of the sample battery cells at the ALS.

