In the quest to realize artificial photosynthesis to convert sunlight, water, and carbon dioxide into fuel – just as plants do – researchers need to not only identify materials to efficiently perform photoelectrochemical water splitting, but also to understand why a certain material may or may not work. Now a team of Foundry users and staff have pioneered a technique that uses nanoscale imaging to understand how local, nanoscale properties can affect a material’s macroscopic performance.
Their study, “Nanoscale Imaging of Charge Carrier Transport in Water Splitting Anodes,” has just been published in Nature Communications. The lead researchers were Johanna Eichhorn and Francesca Toma of Berkeley Lab’s Chemical Sciences Division. Toma is the chair of the Foundry’s User Executive Committee.
Artificial photosynthesis seeks to produce energy-dense fuel using only sunlight, water, and carbon dioxide as inputs. The advantage of such an approach is that it does not compete against food stocks and would produce no or low greenhouse gas emissions. A photoelectrochemical water splitting system requires specialized semiconductors that use sunlight to split water molecules into hydrogen and oxygen.
Bismuth vanadate has been identified as a promising material for a photoanode, which provides charges to oxidize water in a photoelectrochemical cell. This material is a case example in which efficiency should be theoretically good, but in experimental tests it actually has very poor efficiency.
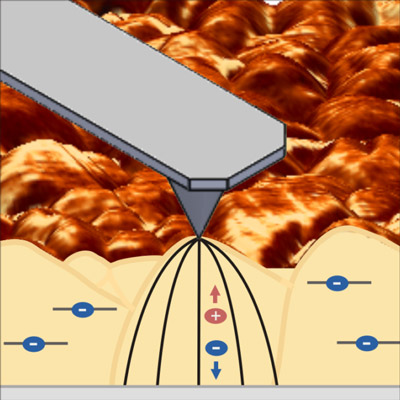
The researchers used photoconductive atomic force microscopy at the Molecular Foundry to map the current at every point of the sample with high spatial resolution. This technique has already been used to analyze local charge transport and optoelectronic properties of solar cell materials but is not known to have been used to understand the charge carrier transport limitations at the nanoscale in photoelectrochemical materials.
The improved understanding of how the bismuth vanadate is working will also allow researchers to synthesize new materials that may be able to drive the same reaction more efficiently. This study builds on previous research by Toma and others, in which she was able to analyze and predict the mechanism that defines (photo)chemical stability of a photoelectrochemical material.

