MOFs – or metal-organic frameworks – are highly customizable porous network solids featuring cages that can come in many sizes and can attract and hold a variety of chemical components, such as carbon dioxide, methane, and hydrogen gases. And it’s this versatile specificity that gives MOFs so much potential for applications in next-generation batteries and in carbon capture, among a growing list. Despite their many positive traits, their open, porous structure – which holds on to electrons – isn’t ideal for applications that require electrons to freely flow with ions (charged particles) through a device to create an electric current.
Now, a team of Foundry users and staff has developed a technique for making an electrically conductive MOF that could also be used to improve the conductivity of other MOFs.
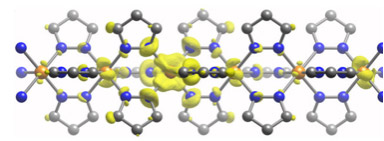
To work around MOFs’ inherently low electrical conductivity, the researchers added a potassium chemical mix to an iron benzenedipyrazolate (BDP) MOF. The extra electrons produced during this reaction can then enter the MOF’s iron center and conduct electricity by hopping along the length of one crystal axis of the rod-shaped crystals. The iron center acts like a wire capable of conducting electricity.
Most MOFs degrade when exposed to potassium, but the iron BDP MOF features robust triangular channels that held up during a series of tests where each reaction boosted the material’s electron count until maximum conductivity for that material was reached, resulting in a MOF that conducts electricity up to 10,000 times better than before it underwent the potassium reactions.
This early demonstration of a highly conductive 3D MOF could bode well for its future use as an all-purpose material for batteries, supercapacitators, and fuel cells. It could also be incorporated into existing composite materials to transform them into porous conductors. And because the potassium-reduced MOF’s organic components are switchable without compromising stability or electron mobility, it could also be used to make different compounds for catalysts and electrolytes.

