Out of the box, MOFs (metal-organic frameworks) look like ordinary salt crystals. But MOFs are anything but ordinary – deep within each crystalline “grain” lies an intricate network of thin, molecular cages that can pull harmful gas emissions like carbon dioxide from the air, and contain them for a really long time. A team of Foundry staff and users have devised a self-assembling “superstructure” made of MOFs and nanocrystals that can store carbon dioxide gas molecules for now, and turn them into useful chemicals and fuels for later.
For years, researchers have tried to combine catalytic nanocrystals and crystalline MOFs into a hybrid material, but conventional methods don’t provide effective strategies for combining these two contrasting forms of matter into one material.
The other problem is that although MOFs and nanocrystals can be mixed in a solution, researchers who have attempted to use methods of self-assembly to combine them have not been able to overcome the natural tendency of these materials to eventually move away from each other – much like the separation you see a few minutes after mixing a homemade salad dressing made of olive oil and vinegar.
Based on thermodynamics-based calculations conducted at NERSC, the researchers predicted that the MOF nanoparticles would form a top layer through molecular bonds between the MOFs and nanocrystals.
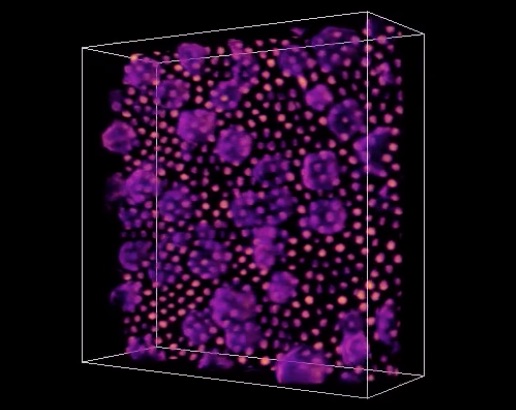
After many rounds of testing different formulations of nanocrystal-MOF molecular bonds, STEM (scanning transmission electron microscopy) images taken at the Foundry’s National Center for Electron Microscopy (NCEM) confirmed that the MOFs self-assembled with the iron-oxide nanocrystals in a uniform pattern.
Using RSoXS at the Advanced Light Source to study the structure, the team made a surprising discovery. In the field of self-assembly, scientists usually expect to see a 2D lattice, but in this case found a “sesame-seed ball” configuration.
The sesame-seed ball configuration is formed by a reaction between the materials that minimizes the thermodynamic self-energy of the MOF with the self-energy of the iron-oxide nanocrystal. Unlike previous MOF/nanocrystal interactions, the molecular interactions between the MOF and the iron-oxide nanocrystal drive the self-assembly of the two materials without compromising their function.
The new design is also the first to loosen rigid requirements for uniform particle sizes of previous self-assembly methods, opening the door for a new MOF design playbook for electronics, optics, catalysis, and biomedicine.

