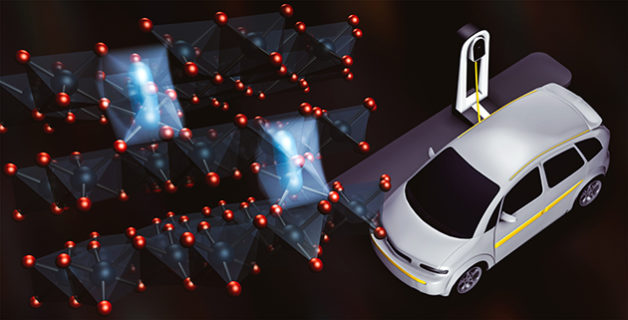
One issue plaguing today’s commercial battery materials is that they are only able to release about half of the lithium ions they contain. A promising solution is to cram cathodes with extra lithium ions, allowing them to store more energy in the same amount of space. But for some reason, every new charge and discharge cycle slowly strips these lithium-rich cathodes of their voltage and capacity.
A new study by Foundry users and staff provides a comprehensive model of this process, identifying what gives rise to it and how it ultimately leads to the battery’s downfall. It was published this week in Nature Materials.
The cycling of lithium through a battery is like a sponge relay, a staple of picnics and Fourth of July barbecues that challenges participants to transfer water from one bucket to another using only a sponge. The more absorbent the sponge, the more water can be squeezed into the second bucket.
Lithium-rich battery cathodes are like super-absorbent sponges, able to soak up nearly twice as many lithium ions as commercial cathodes, packing as much as twice the energy into the same amount of space. This could allow for smaller phone batteries and electric vehicles that travel farther between charges.
Most lithium ion battery cathodes contain alternating layers of lithium and transition metal oxides – elements like nickel or cobalt combined with oxygen. In commercial batteries, every time a lithium atom leaves the cathode for the anode, an electron is snagged from a transition metal atom. These electrons create the electrical current and voltage necessary to charge the material.
Imagine in the sponge relay that with every subsequent soak, the structure of the sponge changes: the fibers stiffen and bundle together, eating up the empty space that makes the material so efficient at absorbing water. Oxygen oxidation does something similar. The authors’ previous study showed that every time lithium ions cycle out of the cathode into the anode, some transition metal atoms sneak in to take their place and the atomic structure of the cathode becomes a little messier. The layered structure essential to the cathode’s performance slowly falls apart, sapping its voltage and capacity.
In this study, the researchers showed that this is because yanking the electron from oxygen makes it want to form another bond and transition metal atoms have to move around to accommodate that bond, changing the atomic structure.

