Scientific Achievement
Users and staff at the Molecular Foundry have found that oxygen oxidation, the process that gives lithium-rich batteries greater capacity, also causes them to fail.
Significance and Impact
This is the first complete picture of the process that causes lithium-rich battery cathodes to fail. Better understanding this process will allow researchers to learn how to control its effects, and lead to better performing batteries for consumer products and electric vehicles.
Research Details
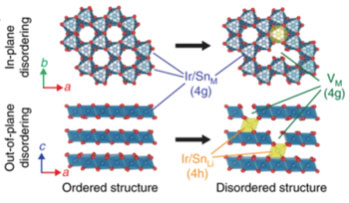
- In lithium-rich batteries, the electrons used for electrical current come from oxygen oxidation. This reaction allows batteries to make use of 90% of the lithium in the cathode, as opposed to 50% in standard batteries, and store more energy.
- Removing electrons from oxygen atoms causes transition metals in the cathode to rearrange themselves, changing the overall structure and reducing energy storage capacity.
- Density Functional Theory (DFT) calculations were crucial for revealing how oxygen oxidation is coupled to changes in the cathode structure.

