
By Brooke Kuei
In 1992, Ron Zuckermann – then a chemist at a biotech start-up and now senior scientist and former facility director of the Biological Nanostructures Facility at Berkeley Lab’s Molecular Foundry – discovered a simple way of making peptoids, a non-natural analog of biopolymers with applications in biomedicine and biomaterials. Peptoids resemble peptides – chains of amino acids – but side chains are attached to the backbone nitrogen atom rather than the carbon atom, allowing peptoids to achieve biological functions such as molecular recognition while at the same time having stability and chemical diversity.
Since its inception almost three decades ago, the peptoid field has attracted researchers from around the world, with the Foundry playing a key role in supporting its community, through the Peptoid Summit and conferences and its state of the art robotic peptoid synthesis facility (see 1, 2). The growth of this field was recently recognized by a two-part special issue on peptoids by Biopolymers, with Part 1 published in April and Part 2 published last month. “The special issue is designed to give people a forum to publish a thematic set of papers about peptoids,” said Zuckermann. “It’s a nice thing to do for the community and will help garner new interest for this growing field.” Both issues were edited by Zuckermann and Foundry user Kent Kirshenbaum, a professor of Chemistry at New York University.
Zuckermann’s early work, which demonstrated that peptoids of defined sequence could be easily made by taking two very simple chemical reactions and linking them together, was originally developed to make short chains for drug discovery. “But I soon realized that this chemistry worked so well that we could make much longer chains,” said Zuckermann. “The accessible chemical diversity of peptoids was so big that the field became about what we should make, rather than what we could make. That’s why I came to the Molecular Foundry: to explore the field of trying to build defined, protein-like nanostructures from biomimetic polymer chains.”
In the years since then, peptoid researchers have sought to merge the fields of polymer science and structural biology. On the one hand, polymer science has been solving the problem of how to link chemical units together in a defined sequence. On the other hand, structural biology has sought to understand the way amino acid sequences encode for protein folding. A large push in the peptoid community has been to develop “the nanoscience of folding” through a combination of biomimetic chemistry, structure elucidation and computer modeling.
A major breakthrough came in 2010 when the Zuckermann group at the Foundry demonstrated that arranging hydrophilic and hydrophobic units (parts that like water and don’t like water) in a specific pattern could pre-determine the structure that a peptoid takes when it folds and self-assembles. By taking advantage of hydrophobic units’ propensity to hide from water, which would collapse the structure in a specific way, the group was able to create the first peptoid nanosheet. “When I first saw a fragment of a nanosheet under the electron microscope, I knew instantly that something special was going on. We cracked the code!,” said Zuckermann. “And that’s where being at the Foundry was so fruitful, because we could use the advanced characterization methods available here and at the ALS to precisely characterize the molecular structure of these peptoid sheets.”
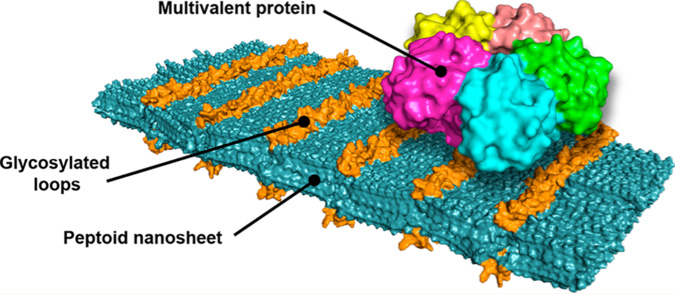
From there, the group moved towards functionalizing the peptoid nanosheet by incorporating a domain with loops in it. “We would put lots of loops on the surface, kind of like Velcro, and these loops can work together to bind something much bigger than the sheet,” explained Zuckermann. These functionalized nanosheets have the potential to recognize things like viruses, bacteria, proteins, and toxins, latch onto them, and potentially neutralize them.
The Biopolymers special issues feature the different ways in which scientists from many fields have addressed the design, characterization, and applications of peptoids. Many of the contributions come from users of the Foundry, such as Gangloff et. al.’s work on examining how peptoids of an exact sequence differ from those that have a distribution of chain lengths and Horn et. al.’s use of the peptoid synthesis method to make a new class of bio-inspired polymers similar to peptoids called “phosphoramitoids” from Part 1. From Part 2, Roe et. al. examines strategies for stabilizing the shapes that peptoid chains can make via side-chain-to-main-chain hydrogen bonding and Spencer et. al. discovers that many known peptoid structures arrange themselves into two regimes of bond angles, suggesting that peptoid structure may actually be more predictable than previously thought.
As Zuckermann reflected on his career in this field, he noted that the beauty of peptoids is how simple their chemistry is, and how approachable they can be for young scientists and non-chemists. The Foundry has helped create a momentum of peptoid users from all over the world, from multiple disciplines, and even across generations. As evidenced by the work in the Biopolymers special issue, the field has reached a place where researchers collectively understand how to design and synthesize functional peptoids.
“The fundamental tool kit is there now – we’re actually just at the beginning of the nanoscience of folding,” said Zuckermann. “I’m personally really happy that we have things like the special issue, the Peptoid Summits, and the Foundry, because it’s going to let this field continue to the grow.”

