Adapted from this release by Cornell University
A team of Foundry users, working with staff, has discovered a crystalline material with ultralow thermal conductivity – thus, the ability to turn heat into electricity – which could lead to the design of novel energy conversion materials and devices.
Hybrid (organic-inorganic) perovskites are a new class of materials that have shown great potential for improving solar cells because of their ability to cheaply and efficiently convert light into electricity, but little is understood about their ability to conduct heat.
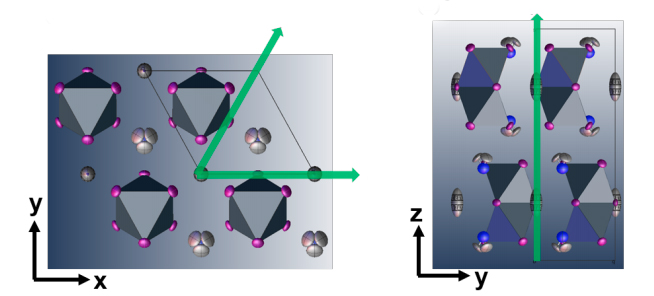
After studying a popular hybrid perovskite and identifying the mechanisms for its low thermal conductivity, Foundry user Zhiting Tian from Cornell University worked with Jeff Urban, director of the Foundry’s Inorganic facility, to identify a hybrid perovskite analogue, methylammonium bismuth iodide (CH3NH3)3Bi2I9, which they hypothesized to have an even lower thermal conductivity because of the unique disconnected structure of its inorganic molecules.
“There are dozens, if not hundreds of perovskites to pursue,” said Urban. “We chose the bismuth system because our prior work studying the thermal transport properties of perovskites indicated that their structure and bonding inclined them toward low thermal conductivity values. Our intuition was that pursuing perovskites composed of heavier atoms (e.g. Bismuth) and lower crystal symmetry could potentially lead very low thermal conductivity materials.”
The thermal conductivity of the material was measured to be .23 Watts-per-meter-Kelvin at 300 kelvins (80 degrees Fahrenheit), among the lowest ever observed in a crystal. The results are detailed in the study “Supercompliant and Soft (CH3NH3)3Bi2I9 Crystal with Ultralow Thermal Conductivity,” published recently in the journal Physical Review Letters.
“Previously we thought only amorphous material can reach very low thermal conductivity,” said Tian, referring to material lacking lattice structure, “but I think this study, along with several recent studies on hybrid materials, may change our view and point us in a new direction.”
One application for materials with ultralow thermal conductivity is in thermoelectrics – devices that can turn heat directly into electricity. A notable example is aboard the Curiosity Mars rover, which converts the heat released from the decay of a radioactive material into electricity. Engineers are hoping to expand the use of thermoelectrics using new materials that make the technology cheaper and more efficient so that they can be used to capture heat from the sun, cars or even the human body.
The perovskite crystals, grown at the Molecular Foundry, were bombarded with high-energy X-rays to measure how the X-rays scattered. The results showed numerous unexpected data points, which all indicated that the material would be soft and possess an ultralow thermal conductivity.
The unique properties have to do with the interactions between the material’s organic and inorganic molecules. Typically, inorganic molecules – defined by their lack of carbon-hydrogen bonds – bond tightly to each other and have strong interactions with adjoining inorganic compounds, but that is not the case with methylammonium bismuth iodide. Instead, the weak electrostatic interactions between molecules provide a difficult pathway for phonons to travel, giving the material a poor ability to transport heat.
The work provides valuable benchmark data of thermal transport properties for organic-inorganic perovskites, and will help in the design of new materials for energy applications.

