This post has been adapted from Berkeley Lab Newscenter
How a liquid interacts with the surface of a solid is important in batteries and fuel cells, chemical production, corrosion phenomena, and many biological processes.
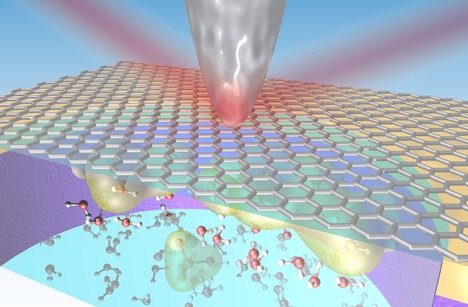
To better understand this solid-liquid interface, Foundry users working with staff developed a platform to explore these interactions under real conditions (“in situ”) at the nanoscale using a technique that combines infrared light with an atomic force microscopy (AFM) probe. The results were published in the journal Nano Letters.
The team explored the interaction of graphene with several liquids, including water and a common battery electrolyte fluid. Graphene is an atomically thin form of carbon. Its single-layer atomic structure gives the material some unique properties, including incredible mechanical strength and high electrical conductivity.
Researchers used a beam of infrared light produced at the Advanced Light Source and they focused it at the tip of an AFM probe that scanned across a section of graphene in contact with the liquids. The infrared technique provides a nondestructive way to explore the active nanoscale chemistry of the solid-liquid interface.
By measuring the infrared light scattered from the probe’s tip, researchers collected details about the chemical compounds and the concentration of charged particles along the solid-liquid interface. The same technique, which revealed hidden features at this interface that were not seen using conventional methods, can be used to explore a range of materials and liquids.

