by Clarissa Bhargava
Millions of people witness solvation phenomena every day – we make solutions whenever we season a pot of water with salt or add sugar to tea or coffee. After a few stirs, these additions readily dissolve, surrounding themselves with solvent molecules until they are evenly dispersed.
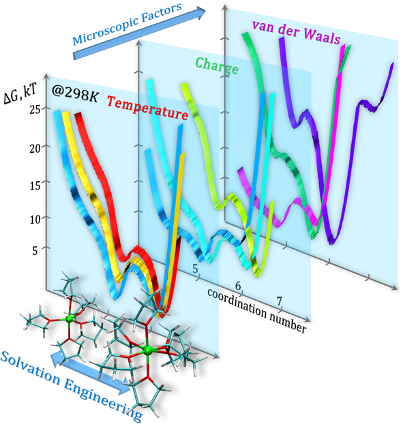
Many solutions only have one stable ion-solvent arrangement. In the case of salt (NaCl), each ion is happiest when surrounded by an average of between 5 and 6 water molecules (H2O). If you were to crowd more water molecules around a single ion, or if you were to pull some away, it would require free energy to do so, and, if left to its own devices, the system would automatically seek to get rid of that extra energy and reassert its ideal coordination number.
Complex solutions are key ingredients in everyday items like batteries, cleaning products, anti-rust coatings, and medicines. In each case, the solvation configuration directly impacts how well the solution plays its part.
In a recent study, scientists from the Joint Center for Energy Storage Research (JCESR) working at the Molecular Foundry, Artem Baskin and David Prendergast, discovered that solutions of free divalent cations (ions with an elementary charge of +2) can be stabilized in several distinct configurations – not just one. Their work offers a playbook to achieve low- and high-coordination configurations by adjusting factors like the solvent makeup and the solution temperature.
To reveal this multiplicity, the team carefully explored the thermodynamic stability of different solvation configurations for several free divalent cations, their pairing with an oppositely-charged ion (anion), and their neutral aggregates in organic solvents. These simulations employed free-energy sampling techniques to drive the system from one configuration to another and monitor the energetic cost.
One of the variables that Baskin and Prendergast considered was the effective charge of the cation. For example, when dissolved, magnesium usually has a +2 charge (Mg2+), but this is not the full picture. An ion’s effective charge can vary based on the environment; the ion-solvent arrangement is strongly influenced by electrostatic interactions. In turn, these forces are influenced by whether the ion’s electron distribution is symmetric or lopsided (polarity), the degree of electron-sharing (covalency), and the charge difference between the ion and the solvent (ionicity).
The JCESR team found that tuning the effective cation charge, which can be done by choosing a solvent for its ability to hybridize electronically with the cation, allows them to change the coordination number (5-fold vs. 6-fold). A higher effective charge stabilizes the configuration with a higher coordination number.
Another option is to adjust the temperature. The simulations demonstrate that raising the temperature stabilizes the lower-coordinated solvation states (and eliminates the higher-coordinated states). For the complex cation MgTFSI+, that stabilization is due to an increase in entropy (i.e., disorder) when solvent molecules are no longer tethered within the cation coordination sphere (i.e., lower coordination). It could appear counterintuitive that the number of configurations available at a given temperature depends so strongly on the entropic contribution of the solvent’s degrees of freedom. Baskin and Prendergast’s view is that this is a particular aspect of the interaction of organic solvents (i.e., not water) and highly charged cations.
This study offers general rules for creating solutions where one could take advantage of ion solvation multiplicity. For example, tuning the solvation configuration would enable control over the energetics of metal plating and stripping in electrochemistry, charge transfer kinetics in surface coatings that prevent corrosion, and homogenous catalysis involving complex ions.
What’s next? The authors are exploring how this behavior is modified near electrode surfaces in batteries, a crucial factor in battery performance. The authors also look forward to seeing these predicted phenomena confirmed experimentally through nuclear magnetic resonance (NMR), dielectric relaxation, or through anisotropy decay measurements with polarization-resolved infrared (IR) spectroscopy.
Read the paper:
Baskin, Artem and Prendergast, David. J. Phys. Chem. Lett. 2020, 11, 21, 9336–9343. DOI: 10.1021/acs.jpclett.0c02682

