Scientific Achievement
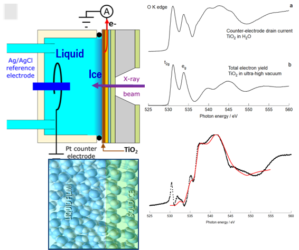
Demonstrated that the first few (~4) layers of water at the TiO2-water interface are structured like ice, a finding that helps understand ionic distribution, ion insertion, and solvation phenomena in oxide materials
Significance and Impact
Advancing the understanding of the molecular structure of aqueous solid-liquid (S-L) interfaces is key to improve & control corrosion, desalination, and electrochemical reactions, where ions have to diffuse though solvent layers near interfaces
Research Details
- Near Edge X-Ray Absorption spectroscopy was used in the electron-yield mode collected at the sample electrode. The short mean free path of the electrons ensures a spatial resolution of few nm
- The O K-edge spectra of the TiO2 film (6.6 nm) acting as the working electrode and that of nearby water reveal the ice-like structure of the first few water layers

