By Matt Lundy
Electric vehicles (EVs) are a burgeoning technology that will only continue to grow with time. As demand increases, researchers are working hard to develop batteries with higher energy density that can support longer-range and cargo-holding capabilities. However, as batteries evolve to store more energy for larger vehicles, prioritizing safety remains paramount. Fortunately, researchers are also working to increase the safety of EV batteries through a variety of means. One method for improving safety is to replace combustible liquid electrolytes found in today’s batteries with solid electrolytes. This switch not only reduces the risk of fires but also contributes to greater efficiency in recycling processes.
In a paper published in Science Advances, a team of researchers led by Foundry staff scientist Brett Helms explored the benefits of solid electrolytes in EV batteries, also known as solid-state batteries or SSBs. The study presents a new kind of SSB that enables direct cathode recycling.
Recycling current, liquid-based EV batteries is a laborious process. It requires complex separations to refine the metal salts before they can be turned back into high-performing cathodes for new batteries. However, switching from a combustible liquid electrolyte to a noncombustible electrolyte imposes its own challenges – like needing to separate the metals making up the electrolyte from those of the cathode. Finding a new way to build SSBs that makes it easier to recycle battery cathodes will help minimize the amount of new materials that need to be extracted from mineral resources for future battery production.
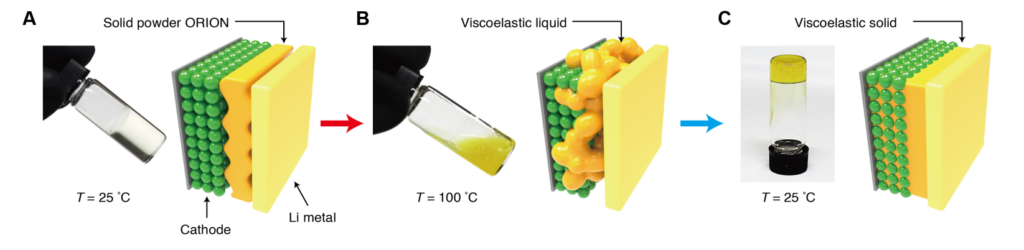
The new SSB works by making use of a special solid electrolyte, called organo ionic (ORION) lithium-ion conductors. These ORION conductors were specifically developed for certain properties that make them ideal candidates for SSBs.
While an ORION-filled battery is operating throughout its life-cycle, temperatures stay between –40 to 45°C. At these temperatures, the ORION conductors are viscoelastic solids, which allows them to help the SSB operate normally. But after a battery reaches its end-of-life, the ORION conductors can be heated and then dissolved, “allowing recyclers to recover the cathode particles directly, which can immediately go back into electrode fabrication and battery assembly,” said Helms.
The development of the ORION conductors sets SSBs on a more viable path of practical adoption. The unique properties of the conductors help SSBs avoid a major pitfall, intensive recycling processes, thereby opening the door for a new advancement in the field of EV batteries.
The process of developing and testing the ORION conductors was made possible by facilities at the Foundry and other Berkeley Lab research centers. The Foundry’s Organic Facility was pivotal in the synthesis and capability characterization of the conductors. Computational resources and methods for studying coordination and solid-solvation in the conductors took place at the National Energy Research Scientific Computing Center facility with software tools developed as part of the Materials Project.
Interested in Becoming a Foundry User?
Join our collaborative, multidisciplinary environment.
Learn more >
The team made use of battery testing infrastructure in the General Purpose Labs to assess the performance and efficiency of the new battery designs. Also, further insight into the performance and physical attributes of the ORION conductors was made possible through rheological analysis capabilities at the Materials Sciences Division and X-Ray capabilities at the Advanced Light Source.
Through these tools and capabilities, the team was able to formulate effective ORION conductors that succeeded in being easily recyclable. Former Foundry postdoctoral associate and lead author Jiwoong Bae noted that “by permitting direct cathode recycling, we avoid having to waste all of that energy in recycling batteries to their base metals and then remanufacturing each component all over again.”
Recycling was made easier, and battery quality did not significantly suffer. According to the study’s findings, the recycled material worked with high efficiency when placed into new batteries. The study’s results show that refurbished cells recover 90% of their capacity and sustain it in their second life, with fade rates comparable to their first life. Recycled battery parts are almost like new.
With easy and efficient recycling, batteries can be manufactured with less new materials. This reduces both the need to mine for more materials and the amount landfilled for metals that are difficult to recover from EV batteries.
Looking to the future, Helms wants to apply the ORION conductors to new scenarios. “We plan to expand our scope to sodium-ion and sodium metal batteries and understand how the reconfigurability of ORION conductors opens the door to conversion cathode materials that experience large volume changes during cycling,” said Helms. “Not only are these emerging chemistries more sustainable in their supply chains, but specific designs enable batteries with specific energy densities four-times that of today’s lithium-ion chemistry.”

