By Renée Haran
Big biological molecules rule in the hidden world of cells. Their forms and functions are inseparable. Technological breakthroughs in the last decade, such as the development of cryo-electron microscopy, have led to a deeper understanding of life’s tiny but mighty building blocks.
Still, much about the cell cycle remains mysterious. That includes the physical structure of chromatin – a DNA protein complex that regulates cell transcription and replication – and how it transitions between cell phases.
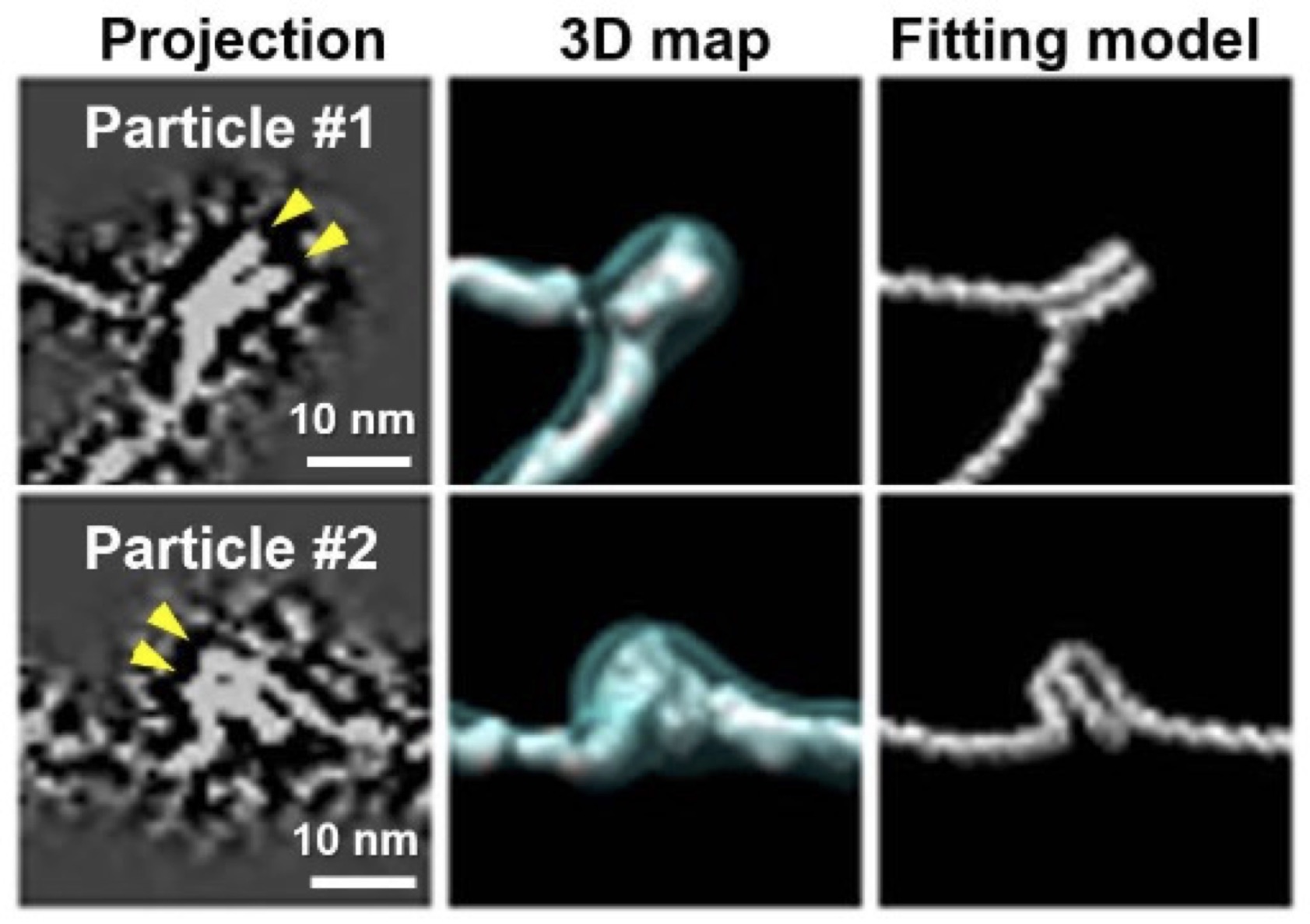
For the first time, a team of Foundry staff and users have determined the structure of individual nucleosome arrays via direct imaging without applying structural averaging. Nucleosome arrays are chromatin’s fundamental units, containing nucleosome discs and linker DNA.
Historically, leading scientists have thought that the 3D imaging of individual macromolecules was impossible. “It was believed to be a dead end due to weak signal and radiation damage limits,” said Ren, Foundry staff scientist in the Imaging and Manipulation of Nanostructures facility, who co-led the team along with collaborators from UC Berkeley (UCB) and Pacific Northwest National Laboratory (PNNL). Their findings appeared in Nature Communications.
Imaging the structure of macromolecules typically requires an averaging process; a small percentage of similar particles are preselected from a large pool and then averaged to produce a static 3D image. While this tried-and-true technique allows for the determination of high-resolution structures, it proves challenging to image the 3D structures of particles like nucleosome arrays due to their dynamic, flexible structures.
Interested in Becoming a Foundry User?
Join our collaborative, multidisciplinary environment.
Learn more >
Under low ionic strength conditions, the researchers combined cryogenic Electron Tomography (cryo-ET) – the flash freezing, thinning, and imaging of a biological sample with an electron microscope as it tilts along an axis – with Foundry-developed computational methods for 3D reconstruction and restoration, known as Individual-Particle Electron Tomography (IPET). They also used atomic force microscopy (AFM) for validation.
This combined approach has illuminated how tetranucleosome arrays undergo intra-array compaction. That’s compaction within itself before entering the early stages of liquid-liquid phase separation (LLPS). LLPS has gained acceptance as a compelling mechanism for the formation of membrane-less cell structures.
By lowering ionic strength conditions in their method, the team slowed the incubation of intra-nucleosomal array structures down to roughly five- and twentyfold. The slower pace allowed them to visualize previously unseen structures.
Like other large biomolecules, chromatin consists of freely rotating DNA chains that bend into different positions. Known as conformations, the forms are often biologically active and take shape without breaking any bonds. Chromatin switches between its active or inactive states depending on its shape: when in its loose “beads on a string” form, chromatin becomes active; when tightened up, inactive.
While averaging provides higher-resolution images than IPET, its view is narrow. IPET allows researchers to “see the overall picture” of molecular conformations, Ren said. “We see a much wider window that includes low-population and high-energy state conformations.”
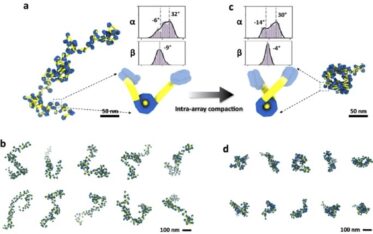
With the benefits of an expanded view, the team also identified two critical components in determining chromatin array conformation: the angles between linker DNA entry and exit points and the corresponding tangents to their nucleosomal discs. Changes in the linker DNA angles were found to regulate intra-array compaction levels, resulting in a variety of tetranucleosome conformations.
Further, the researchers investigated the effect of H1 on intra-array compaction. H1 is a histone protein component of chromatin and a known catalyst for phase separation. Researchers found that H1 induced a “closed” form in DNA linkers. Its presence promoted nucleosomal disc invasion by foreign DNA linkers from neighboring discs, possibly increasing chromatin networking and compaction.
In their previous 2022 publication, the researchers investigated the early stages of tetranucleosome phase separation. Using a similar method, they demonstrated that phase separation occurs in two steps: “spinodal decomposition” followed by the formation of nuclei, which eventually mature into jellylike spherical droplets. Their recent findings explored the initial stages of intra-array compaction before LLPS, widening the picture even further.
While there is much more to discover about the secret lives of cells, the researcher’s findings shed more light on how chromatin moves from intra-array compaction to interphase and metaphase, enhancing human understanding of DNA’s inner workings and the cell cycle.
“Proteins control life. Their 3D structures control molecular interactions,” said Ren. “Once you know the structure, you know their physical process.”

