By Claire Tsai
Bacteria have long harnessed protein-based, self-assembling organelles called bacterial microcompartments (BMCs) to encapsulate catalysts and compartmentalize specific metabolic pathways. A team of Foundry users have recently developed a rapid mechanism for inducing self-assembly and loading of BMCs in vitro, a breakthrough with far-reaching potential in biocatalysis, artificial photosynthesis, and bionanotechnology.
“Nature has evolved a robust process for making protein shells to house biochemical reactions,” explains Cheryl Kerfeld, a Hannah Distinguished Professor at Michigan State University and the lead investigator for this project. “But if we want to control and co-opt that process, we need fast and precise methods, ideally outside of the cell. Our goal is to build hierarchical catalytic systems based on the BMC shells ultimately leading to multi-room factories where each shell creates a defined microenvironment for catalysis.”
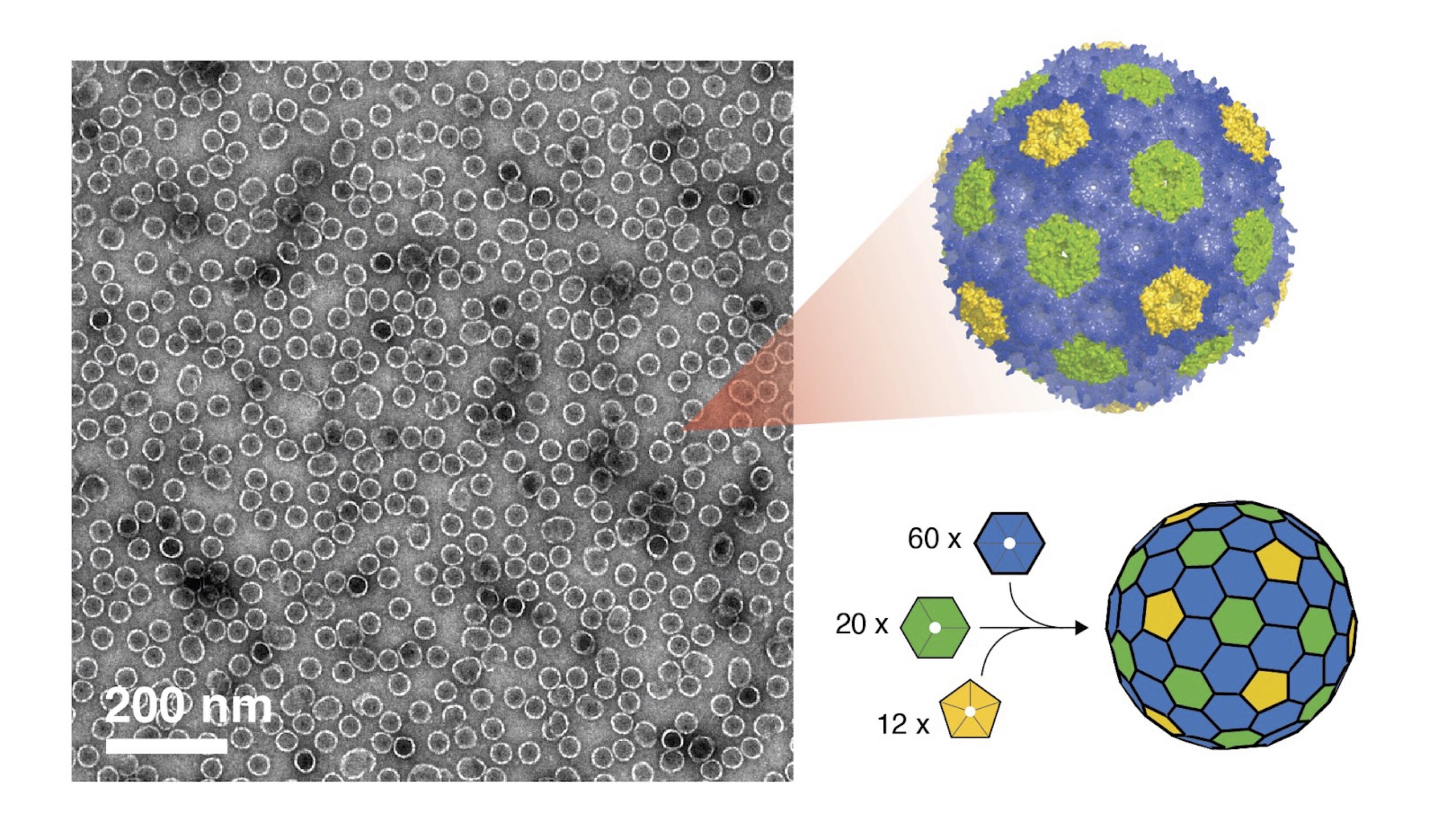
BMCs are composed of a protein shell that encases enzymes and substrates, creating a controlled microenvironment. These BMC shells are formed with various protein tiles—the building blocks of BMC shells—which include hexameric (BMC-H), pseudohexameric (BMC-T), and pentameric (BMC-P) units. When combined, they form shells that are akin to a soccer ball. “Just like how a classic soccer ball has white hexagons and black pentagons, the inclusion of pentagonal proteins introduces the curvature needed to close the shell, similar to how hexagonal and pentagonal panels create the rounded shape of a soccer ball” Dr. Tim Chiang, a researcher at the Lawrence National Berkeley Laboratory (Berkeley Lab) notes.
BMCs have traditionally been expressed and purified through a time and labor-intensive in vivo process, which often does not allow for precise control of shell assembly and can capture unwanted contaminants. The team’s new method uses the chaotropic agent urea—a chemical that temporarily disrupts the weak bonds stabilizing proteins—to kickstart assembly. Researcher and co-first author Kyleigh Range, alongside Dr. Chiang, explains how the process works: “These protein tiles (called BMC-H) naturally stick together through various intermolecular interactions. When we add urea—a ‘chaotrope’ or chemical that disrupts certain non-covalent interactions—it breaks apart these protein sheets into individual pieces.” Once separated, the team mixes these BMC-H tiles with two other shell components (called BMC-T and BMC-P) and the pieces quickly snap together to form complete shells. “This method assembles shells with nearly perfect efficency—a major improvement over older techniques,” Range adds. This approach, akin to assembling a modular LEGO set, allows scientists to bypass living cells and create varied shell types with remarkable speed and precision. Their results were recently published in ACS Nano.
“The method we are using tolerates a huge range of conditions and because it is so robust, we are able to put all kinds of things inside that nature cannot” Kerfeld notes. This robustness allows for precise control of shell composition and the encapsulation of enzymes or other catalytic molecules—biotic or abiotic—under ambient conditions, and in less than an hour. These engineered bacterial microcompartments act as miniature “nanofactories,” efficiently bringing together enzymes and materials within nanoscale spaces to perform highly controlled biochemical reactions, much like tiny production lines inside cells.
Interested in Becoming a Foundry User?
Join our collaborative, multidisciplinary environment.
Learn more >
This interdisciplinary effort, including Michigan State University, Argonne National Laboratory, and the Molecular Foundry as a part of the Center for Catalysis in Biomimetic Confinement (CCBC), a Department of Energy (DOE) Energy Frontier Research Center, leveraged the Foundry’s advanced facilities to develop these shells. Corie Ralston, a Facility Director at the Molecular Foundry at Berkeley Lab explains, “the localization of all this expertise and instrumentation available at the Foundry made it really attractive to be part of the CCBC. The team developed the protein production and shell assembly platform here at the Foundry, and then transferred the technology to Argonne and MSU so that all three locations could produce BMCs.” Advanced instrumentation at the Foundry also proved essential for confirming shell formation. “We used the dynamic light scattering instrument (DLS) on this floor to measure the size of the forming shells,” explained Ralston. Electron microscopy offered an even closer look, “providing direct visualization of the protein shells and confirming their shape and structure beyond simple size measurements,” added Chiang.
Looking ahead, the researchers envision constructing modular, multi-compartment catalytic systems where each compartment maintains a tailored microenvironment to optimize complex biochemical catalysis. “These protein shells represent this hallmark of life. It’s this idea that compartmentalization can serve many different purposes” Chiang notes. This breakthrough not only accelerates fundamental research into bacterial organelles but also promises biotechnological innovations in biosensing, synthetic living systems, and in medicine.
As Kerfeld succinctly puts it, “This work provides a whole new biomaterial that could be used in synthetic biology beyond catalysis, including precision agriculture and even drug delivery.”

