By Harkirat Batth
Understanding how nanocrystals grow, and precisely controlling that growth during their synthesis, is incredibly important for creating better batteries, improving catalysts, and much more. However, traditional measurements of nanocrystals don’t consider the size and shape distribution of single nanocrystals within populations. This gap has long hidden structure-property relationships in nanocrystals, preventing scientists from studying small differences and variabilities. Compounding this challenge is that even the best synthetic chemists can’t make completely uniform batches of nanocrystals.
Now, a team of Foundry staff and users has developed a groundbreaking approach using artificial intelligence that analyzed nearly half a million individual nanocrystals. Their findings, published in the journal ACS Nano, reveal how these tiny particles change shape as they grow, which could improve how we manufacture nanomaterials.
Think of nanocrystals like snowflakes: no two are exactly alike, even when made in the same batch. Traditional measurement methods could only look at large groups of nanocrystals together, like trying to understand snowflake formation by measuring a handful of snow. This “ensemble” approach hid crucial details about how individual crystals developed different shapes and sizes.
“We know there’s always heterogeneity in nanocrystal populations,” said Mary Scott, faculty scientist at the Molecular Foundry’s National Center for Electron Microscopy (NCEM). “There’s always some distribution of size and shape. Even with excellent synthesis, you cannot get the population to be perfectly uniform. One of our big-picture goals for this study was to actually measure that heterogeneity.”
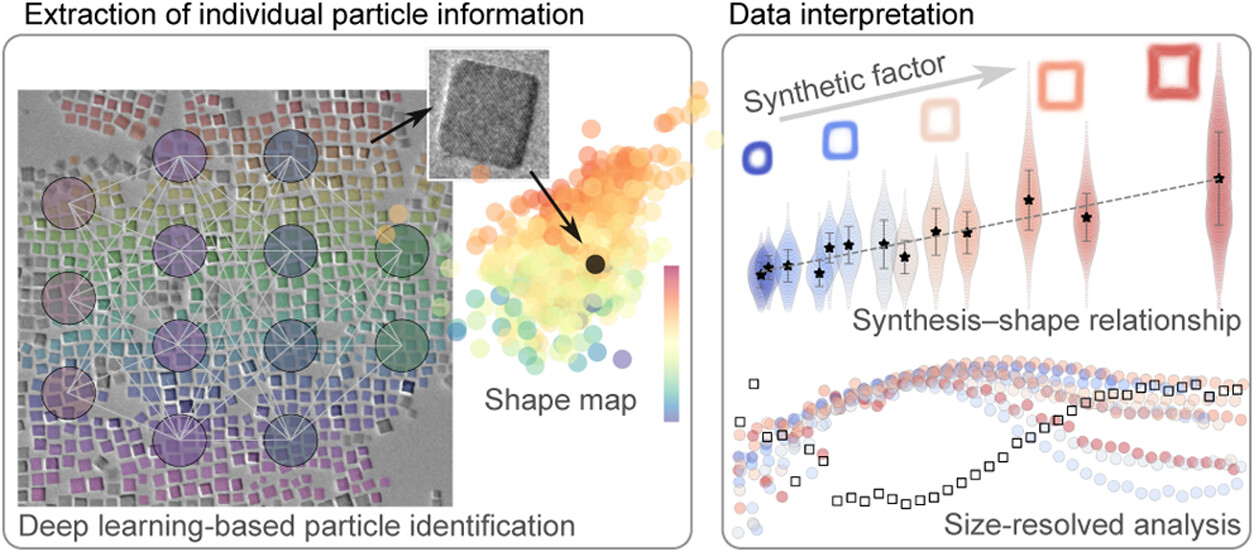
The research team turned to deep learning – the same technology behind facial recognition – to solve this problem. They trained computer algorithms to automatically identify and measure the geometric features of individual nanocrystals, most less than 10 nm across.
Using high-resolution electron microscopy, the research team captured detailed images of over 400,000 cobalt oxide nanocrystals — individually. The AI system analyzed each one, measuring dimensions down to a fraction of a nanometer.
The massive dataset revealed something never seen before: nanocrystals don’t just grow larger, they fundamentally change shape at specific size thresholds. The team discovered that there is a shift in growth trends at a point they named the “onset radius”, the critical transition point in the growth regime where dominant shapes appear at each population.
“A known concept in nanomaterial synthesis is that, depending on the concentrations of different ingredients in the synthesis, you can either be in the thermodynamic or kinetic regime, which each give different shapes. These are the kind of experimental knobs that people would want to be able to control, so that you can get a shape that’s dominated by either regime.” said Scott.
The team’s work gives that control to scientists, so instead of making random batches and hoping for the best, scientists can dial in specific synthesis conditions to produce exactly the nanocrystals they need. The discovery is significant because a nanocrystal’s shape directly determines its properties. Concave nanocrystals, for instance, have more exposed surface area and specific atomic arrangements that make them better catalysts for chemical reactions.
Part of the success of this study lies in researchers combining the use of deep learning tools with high-resolution electron microscopy to extract size and shape data from individual particles. Without the use of deep learning, it would have been much more difficult to get the results the group did.
Interested in Becoming a Foundry User?
Join our collaborative, multidisciplinary environment.
Learn more >
Scott said, “I think we might’ve missed the idea of an onset radius without using deep learning tools. We needed so many nanoparticles to see that inflection point, and had to run the experiment so many times that deep learning tools definitely helped take this experiment further.”
AI and deep learning machine tools will likely shape research, especially materials research, heavily over the coming years. It is creating new possibilities within science and allowing researchers to see and understand things at a level not previously possible. Using it responsibly could enable positive change within the research community.
“I definitely see the use of deep learning when doing electron microscopy, “ stated Scott. “We’re really working within my group to get these tools into the hands of the scientific community.”
All of this work was made possible through existing projects going on at NCEM. “This project was funded by a project called the Electron Distillery; the idea is distilling information from really large data sets. It’s been a great collaborative project led by our facility director at NCEM, Andy Minor, and very successful in actually using deep learning. I think this kind of capability development is exactly what the Foundry is very good at. It’s a great example of being science-driven, and also creating this new capability that we hope other people will soon be able to use in creative ways that we wouldn’t have thought of before.”

