Adapted from this Berkeley Lab Press Release
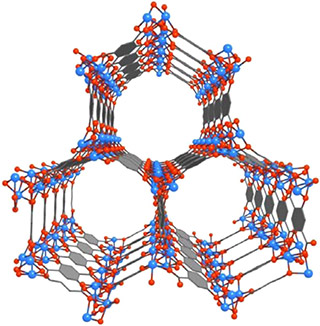
A unique inside look at the electronic structure of a highly touted metal-organic framework (MOF) as it is adsorbing carbon dioxide gas should help in the design of new and improved MOFs for carbon capture and storage. Researchers with the U.S. Department of Energy (DOE)’s Lawrence Berkeley National Laboratory (Berkeley Lab) have recorded the first in situ electronic structure observations of the adsorption of carbon dioxide inside Mg-MOF-74, an open metal site MOF that has emerged as one of the most promising strategies for capturing and storing greenhouse gases.
Working at Berkeley Lab’s Advanced Light Source (ALS), a team led by Jeff Kortright of Berkeley Lab’s Materials Sciences Division, used the X-ray spectroscopy technique known as Near Edge X-ray Absorption Fine Structure (NEXAFS) to obtain what are believed to be the first ever measurements of chemical and electronic signatures inside of a MOF during gas adsorption. In addition to Kortright, the team included Jeffrey Neaton and David Prendergast from the Molecular Foundry’s Theory Facility who assisted with the first principle calculations that provided a theoretical model as to what should happen inside Mg-MOF-74 during adsorption of carbon dioxide.
Carbon dioxide gas released during the burning of coal is one of the primary greenhouse gases responsible for exacerbating global climate change. However, with the world’s largest estimated recoverable reserves of coal, the United States will continue to rely on coal-burning power plants to generate electricity for the foreseeable future. This presents a pressing need to develop effective and economical means of removing carbon dioxide from flues before it enters the atmosphere.
MOFs are molecular systems consisting of a metal oxide center surrounded by organic “linker” molecules that form a highly porous three-dimensional crystal framework. This microporous crystal structure enables MOFs to serve as storage vessels with a sponge-like capacity for capturing and containing greenhouse gases. When a solvent molecule applied during the formation of the MOF is subsequently removed, the result is an unsaturated “open” metal site MOF that has a strong affinity for carbon dioxide.

