Scientific Achievement
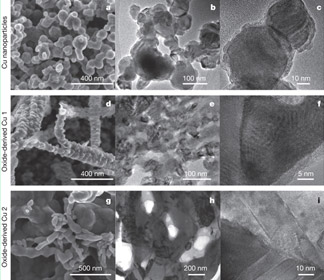
A multidisciplinary team of users at the National Center for Electron Microscopy (NCEM) created copper-derived electrocatalysts that efficiently produce appreciable amounts of ethanol from carbon monoxide (CO) gas at room temperature and pressure.
Significance and Impact
Oxide-derived Cu (OD-Cu) electrocatalysts exhibit a 10x improvement over conventional CO reduction catalysts and demonstrate the feasibility of a two-step conversion of CO2 to liquid fuel at low applied voltages.
Research Details
- Ethanol is a liquid fuel, typically produced by high temperature biomass fermentation, resulting in low production efficiencies.
- While many catalysts can reduce CO2 to CO, copper is one of the few materials capable of converting CO to ethanol. However, conventional copper is very inefficient.
- OD-Cu catalysts form a continuous nanocrystalline framework with well-defined grain boundaries between Cu nanoparticles – revealed by NCEM’s TEAM 0.5 microscope, which suppresses interface delocalization – that are believed to result in the increased catalytic efficiencies.

