Despite its fleeting existence – about 300 nanoseconds – carbonic acid is a crucial intermediate species in the equilibrium between carbon dioxide, water and many minerals. It plays a crucial role in the carbon cycle – the exchange of carbon dioxide between the atmosphere and the oceans – and in the buffering of blood and other bodily fluids. However, the short life span of carbonic acid in water has made it extremely difficult to study. A new study by Berkeley Lab researchers using the Molecular Foundry has yielded valuable new information about carbonic acid with important implications for both geological and biological concerns.
Richard Saykally, a chemist with Berkeley Lab’s Chemical Sciences Division, worked with the Foundry’s David Prendergast, to lead a study that produced the first X-ray absorption spectroscopy (XAS) measurements for aqueous carbonic acid. These XAS measurements, which were obtained at ALS, were in strong agreement with supercomputer predictions obtained at the NERSC.
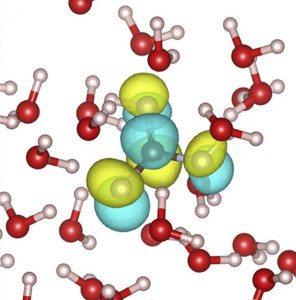
Prendergast led the effort to use molecular dynamics simulations and first principles density functional theory to model and interpret the XAS measurements to simulate how carbonic acid is solvated by water. This was then converted into a predicted XAS absorption spectrum that was directly compared with experimental measurements.

