Molecular Foundry users have developed a new electrocatalyst that can directly convert carbon dioxide into multicarbon fuels and alcohols using record-low inputs of energy. The work is the latest in a round of studies coming out of Berkeley Lab tackling the challenge of creating a clean chemical manufacturing system that can put carbon dioxide to good use.p
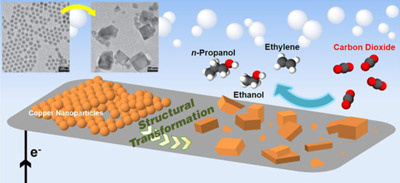
In the new study, published this week in the Proceedings of the National Academy of Sciences, a team led by Foundry user Peidong Yang discovered that an electrocatalyst made up of copper nanoparticles provided the conditions necessary to break down carbon dioxide to form ethylene, ethanol, and propanol.
All those products contain two to three carbon atoms, and all are considered high-value products in modern life. Ethylene is the basic ingredient used to make plastic films and bottles as well as polyvinyl chloride (PVC) pipes. Ethanol, commonly made from biomass, has already established its place as a biofuel additive for gasoline. While propanol is a very effective fuel, it is currently too costly to manufacture to be used for that purpose.
To gauge the energy efficiency of the catalyst, scientists consider the thermodynamic potential of products – the amount of energy that can be gained in an electrochemical reaction – and the amount of extra voltage needed above that thermodynamic potential to drive the reaction at sufficient reaction rates. That extra voltage is called the overpotential; the lower the overpotential, the more efficient the catalyst.
The researchers characterized the electrocatalyst at the Molecular Foundry using a combination of X-ray photoelectron spectroscopy, transmission electron microscopy, and scanning electron microscopy.
The catalyst consisted of tightly packed copper spheres, each about 7 nanometers in diameter, layered on top of carbon paper in a densely packed manner. The researchers found that during the very early period of electrolysis, clusters of nanoparticles fused and transformed into cube-like nanostructures. The cube-like shapes ranged in size from 10 to 40 nanometers.
This latest study exemplifies how carbon dioxide reduction has become an increasingly active area in energy research over the past several years. Instead of harnessing the sun’s energy to convert carbon dioxide into plant food, artificial photosynthesis seeks to use the same starting ingredients to produce chemical precursors commonly used in synthetic products as well as fuels like ethanol.

