Living organisms possess an unparalleled talent for synthesizing complex molecules with high selectivity, while non-living materials comprise the most efficient energy collection systems. Scientists have sought to unite these complementary strengths by electrochemically coupling separate microbial and inorganic catalysts to form biohybrid systems. Such biohybrid systems are attractive as a sustainable technology to produce fuels and high-value chemicals using renewable energy.
But, a fundamental challenge in designing biohybrid systems is that the environments that support optimal function of living cells and inorganic materials are chemically incompatible, resulting in toxicity, corrosion, or efficiency-degrading cross reactions. To date, the approach has been to keep the biological and abiotic components physically separated by macroscopic (millimeter to centimeter) distances. However, this exacts a high cost in terms of efficiency due to resistance losses (on the order of 25 percent of the cell voltage) caused by ion transport between the components, making scale-up to commercially relevant levels impractical.
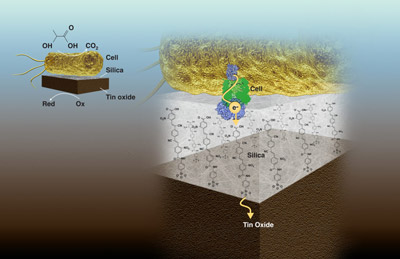
Now, a team of Foundry staff and users has developed a novel nanoscale membrane embedded with molecular wires that simultaneously chemically isolates, yet electrochemically couples, a microbial and an inorganic catalyst on the shortest possible length scale. This new modular architecture, described in a paper recently published in Nature Communications, opens up a large design space for building scalable biohybrid electrochemical systems for a variety of applications, including electricity generation, waste remediation, and resource recovery, in addition to chemical synthesis.
As a proof-of-concept, the researchers electrochemically coupled Shewanella oneidensis, an anerobic bacterium notable for its ability to reduce heavy metals via cytochromes present on the outer membrane, to an inorganic catalyst, tin dioxide. The 2-nanometer-thick silica membrane contains polymers that have functional cyano and nitro chemical groups that transfer electrons, enabling current flow while blocking oxygen and other small molecule transport.

