This article has been adapted from this ALS science highlight.
The last decade has seen extraordinary advancements in electron microscopy, enabling researchers to image materials at increasingly high resolutions. This type of high-resolution imaging, which allows us to “see” structures
Lithium-ion batteries are ubiquitous, yet their performance pales in comparison to that predicted of the long-sought holy grail—a battery with an anode of pure metallic lithium. After decades of study, practical constraints and a lack of fundamental understanding of lithium’s chemistry continue to prevent the full realization of its potential.
A combination of factors has plagued attempts to pin down lithium’s relevant electronic structure. First, metallic lithium is highly reactive and oxidizes almost immediately, contaminating results with data from lithium oxide. Previous studies have yielded contradictory results: K-edge x-ray absorption spectra of metallic lithium have differed significantly from the electron energy loss spectrum, which in turn has differed from the hard x-ray Raman spectrum. Second, although lithium K-edge absorption spectroscopy is the most direct way to probe the element’s electronic structure, it requires a very low x-ray energy range (55–65 eV) and very high energy resolution, a combination that is not commonly available at x-ray facilities. The non-trivial nature of spectroscopic probes of the very active alkali metals led to debates over 50 years ago, which were eventually clarified through careful experiments by Neville Smith, a former ALS science deputy, during his doctoral work.
In addition, despite being a metal, the small number of electrons per lithium atom leads to a stronger-than-expected many-body response to x-ray excitation—a phenomenon that is challenging to computationally model with popular methods. Furthermore, the precision and validity of the theoretical calculations have suffered from the lack of reliable experimental results, so it has been hard to achieve a quantitative level of comparison between the theory and experiment.
Now, using the ultra-high-resolution (<10 meV) soft x-rays offered by the ALS and novel theoretical approaches developed at the Molecular Foundry, researchers have uncovered new information about the electronic structure of lithium and explained the contradictions in previous studies.
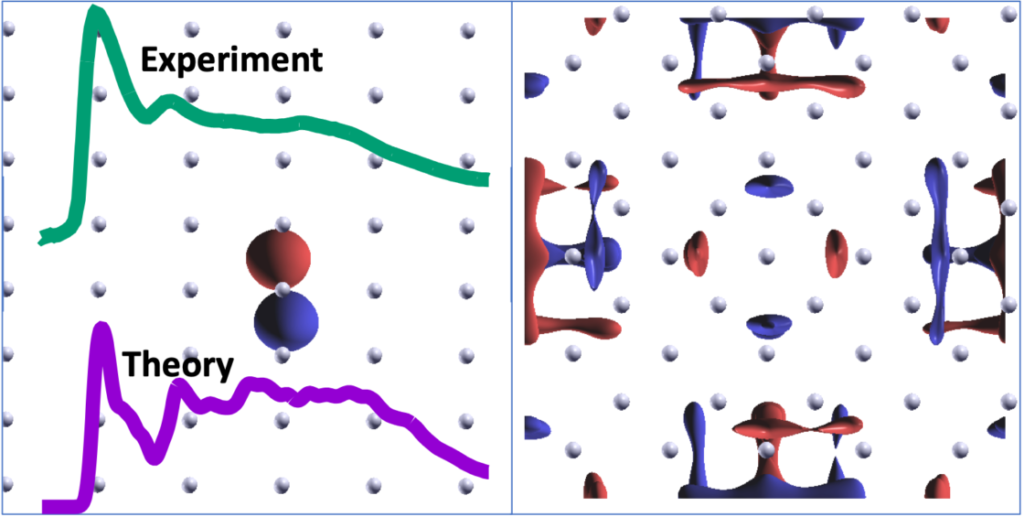
To investigate the K-edge absorption spectrum, the researchers used ALS to explore two different in situ preparation methods—cleaving and evaporation in ultra-high vacuum (UHV). These techniques were used to mitigate oxidation, allowing the team to measure the pristine metal.
Interested in Becoming a Foundry User?
Join our collaborative, multidisciplinary environment.
Learn more >
The spectrum for the freshly cleaved sample showed a strong peak at 55.6 eV, which was either absent or severely suppressed in previous studies, most likely due to oxidation of lithium’s surface. The team continued to take measurements at regular intervals. After only 20 minutes in the UHV environment, features of Li2O began to appear, diminishing, and eventually obscuring, the peak associated with the freshly cleaved sample.
The oxygen contamination associated with real-world experiments shouldn’t be a problem in silico, but oddly, the absorption peak at 55.6 eV was also missing from previously reported theoretical predictions of pure lithium. These had relied on single-particle approaches based on density functional theory. To more accurately describe the x-ray excitation of lithium metal atoms’ core electrons, the researchers developed a new computational treatment that explicitly accounted for the many-body response of all valence electrons to the core excitation, and they were able to replicate the 55.6 eV peak. They found that the prominent peak contains significant contributions from multiple near-degenerate excitations, some of which are forbidden within a simplified single-particle framework.
The team extended their successful experimental and theoretical approaches to a series of lithium salts, establishing a solid benchmark of experimental spectra and quantitative theoretical calculations for studying lithium chemistry.
Read this highlight on the ALS website.

