
Staff Scientist, Biological Nanostructures
510.486.4299
Biography
Education
Dr. Ajo-Franklin has been a Staff Scientist at the Molecular Foundry since 2007. Before that, she received her Ph.D. in Chemistry from Stanford University with Prof. Steve Boxer and was a post-doctoral fellow with Prof. Pam Silver in the Department of Systems Biology at Harvard Medical School.
Dr. Ajo-Franklin is fascinated by the incredible, diverse functionality of biological molecules and nanoassemblies, and seeks to engineer these complexes and their host organisms to address global challenges in energy and the environment.
Research Interests
Dr. Ajo-Franklin’s research creates engineered bioassemblies–electron nanoconduits–that direct the flow of charge between living cells and non-living materials. Though a combinatorial approach, she has established an unparalleled ability to genetically program the enormously complex biosynthesis of these electron nanoconduits and to quantitatively characterize their function at the biological-inorganic nanointerface. These capabilities have allowed her to discover design principles that govern the emergent processes of nanoconduit assembly and function. In future work, she will expand the combinatorial space through designed genetic variants and adaptive evolution approaches to dissect fundamental controls on charge transfer at this hybrid nanointerface.
Projects
Electron Nanoconduits to Electrically Connect Living and Non-Living Systems
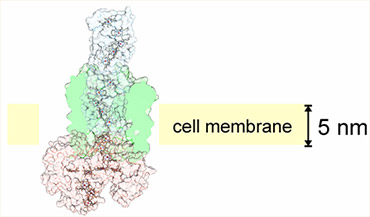
Cellular-electrical connections would enable devices to combine the specialties of the living world and the non-living technological world. This new class of smart, self-renewing nanostructured systems has the potential to revolutionize environmental sensing and energy harvesting applications and to open new avenues to program cellular behavior. Building towards this vision, the long-term objectives of this research are to develop understanding of the principles which govern electron flow across living/non-living interfaces and to use those principles to create electron transfer pathways between individual living microbial cells and non-living electrodes. The overall strategy is both to explore how naturally-occurring microbes achieve electron transfer to inorganic surfaces and to use genetic and materials surface engineering to create new ‘domesticated’ hybrid cell-electrode systems. Read full research papers: 1, 2
Engineering Self-Assembly and Function of S-layer Nanoarrays
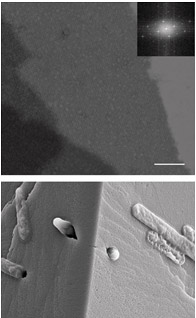
S (surface)-layer proteins form crystalline lattices on the outsides of many bacteria and archaea. These nearly ubquitous structures play a number of crucial biological roles: they serve as structural scaffolds, effect selective transport of ions and proteins, serve as templates for mineralization, and protect against phagocytosis.
The overall goal of our research is to develop ways of functionalizing these scaffolds and to predictively understand S-layer self-assembly, so as to design and create nanostructures scaffolds with novel functions. This research area is a collaborative effort with Steve Whitelam in the Theory Facility. Read full research paper
Ionic Transport Across Lipid Bilayers on Nanomaterials
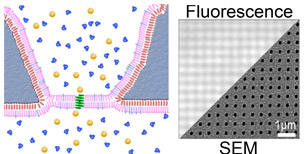
The lipid bilayer membrane serves as the barrier that separates cells from their environment and thus, critically regulates material and information flow into biological systems. As part of several collaborative user projects, we are exploring means to integrate charge transporting proteins in lipid bilayers with 1D and 2D nanostructures. These hybrid nanostructures supply an excellent materials platform for building devices that integrate human-made and biological structures. Read full research papers: 1, 2

