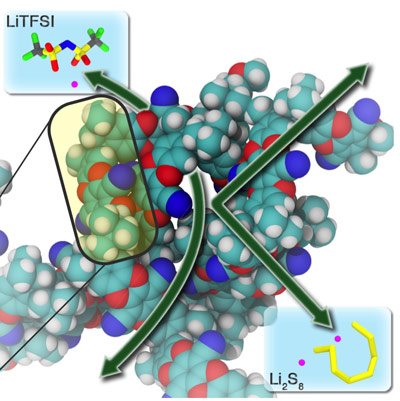
Lithium-sulfur batteries, which store electrical energy by transferring electrons to or from a sulfur electrodeare well poised to provide high-density, long-term and low-cost electrochemical energy storage. The potential of lithium-sulfur batteries, has yet to be fully realized, however, due to the uncontrolled migration of soluble sulfur species through the membrane that separates the electrodes. This crossover of polysulfides reduces battery efficiency and lifetime.
Molecular Foundry staff and users in the Organic and Theory Facilities have combined to find a solution to the polysulfide crossover problem with the development of a membrane made from polymers of intrinsic microporosity (PIMs). PIMs feature pore sizes of less than one nanometer in diameter, compared to the 17 nanometer pore size of typical membrane separators. This substantially smaller pore size provides highly selective control over the ions transported through the membrane. Smaller ions, like lithium and sodium are allowed to pass through the membrane while larger polysulfides are blocked. When integrated into lithium-sulfur cells, PIM membranes proved 500 times more effective at blocking the unwanted crossover of polysulfide ions than conventional membranes.
Guided by theoretical calculations, the team developed and applied PIMs as a membrane platform for achieving high-flux, ion-selective transport in non-aqueous electrolytes. The PIMs are synthesized in a single step and easily cast into large-area sheets with well-controlled pore structure and pore chemistry.

