In most solid, crystalline materials, atoms are arranged in “grains” which are regions where atoms have the same local arrangement. In between these grains, we find grain boundaries where two grains meet.
Grain boundaries are important for many reasons. For example, they strongly affect the mechanical strength of a material, they can dramatically speed up the diffusion of other atoms through the material, and they usually have much lower electrical conductivity than the rest of the grain.
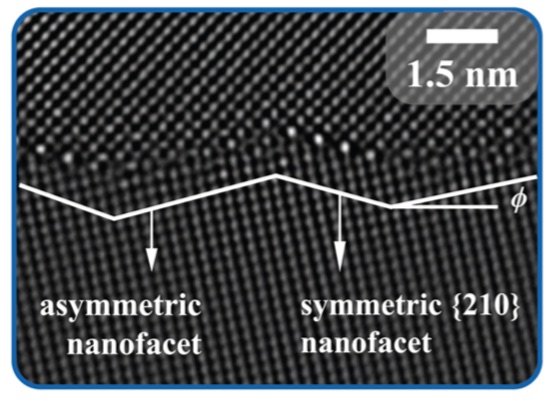
In a study recently published in Physical Review Letters, a team of Molecular Foundry users and staff added silver to a grain boundary in a copper material and directly observed a grain boundary transition deliberately caused by modifying the chemistry of the material. They unambigiously showed that silver segregation at these grain boundaries causes a “nano-faceting” transition where the boundary splits into alternating symmetric and asymmetric nano-facets.
Previously, it’s been shown that forcing a change to the grain boundary, by changing temperature, pressure, or elemental composition, caused an abrupt change to a material’s properties. However, in most cases, researchers did not know what the new atomic structures of the altered grain boundaries were.
The team used scanning transmission electron microscopy (STEM) at the Foundry’s National Center for Electron Microscopy (NCEM) to image the atomic structures, and molecular dynamics simulations to model the transformation and explain why it occurs.
The researchers believe this is a fairly general phenomenon that can happen whenever a grain boundary is close to a “symmetric” orientation where the boundary can absorb many of the solute atoms.

