Understanding the interfaces where solids and liquids meet is key to controlling a wide range of energy-relevant processes, from how batteries store energy to how metals corrode, and more. However, there are many unanswered questions around how these processes work at the atomic or molecular scale.
Now a team of Foundry staff and users have explored such interfaces and found what they describe as a treasure trove of unexpected results that expands our understanding of working interfaces and how to probe them.
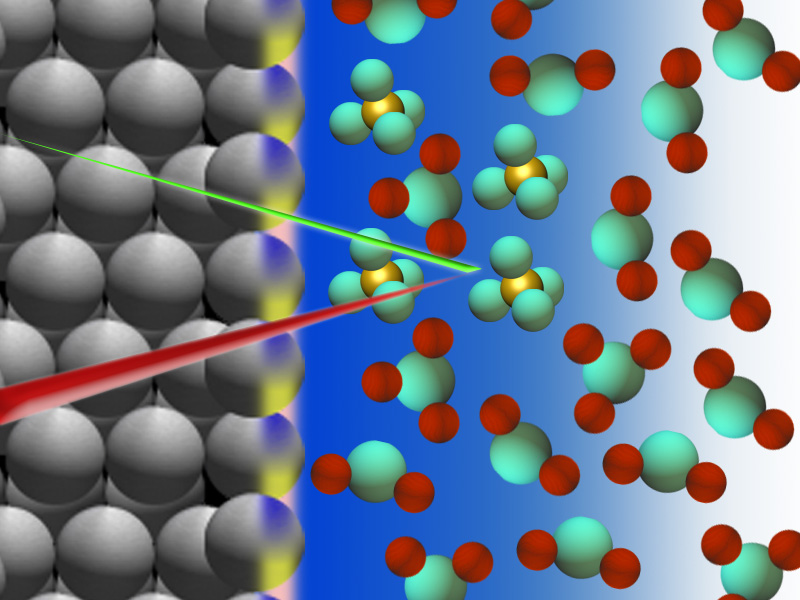
They deployed a powerful X-ray technique to detect the hidden “fingerprints” of various chemical species that collect just above the surface of a platinum electrode immersed in sulfuric acid. They then used supercomputer simulations to make sense of these measurements. This first-of-its-kind study of the molecular structure of the platinum-sulfuric acid interface was recently published in the Journal of the American Chemical Society.
Just before oxygen should be produced, it had long been believed that the surface of the metal electrode starts to corrode or oxidize. What the Berkeley Lab team found challenges the conventional understanding of this electrochemical interface. They found no evidence for the presence of platinum oxide at this stage of the reaction. Instead, the team’s measurements were interpreted as indicating elevated concentrations of sulfate ions near the platinum surface – concentrations that are much higher than those found in the liquid far from the electrode.
The findings will have a direct impact in scientists’ ability to understand wetting, corrosion, membranes, and electrochemical phenomena. Now that the Berkeley Lab researchers have proven that rust isn’t always a foregone conclusion, they hope to further their work by using X-ray spectroscopy to study how corrosion occurs in copper or iron.

